
Description:Expressed in E.coli and purifed to high homogeneity with high protease activity, used to cleavage of SUMO fusion proteins.
Description
SUMO Protease is a recombinant fragment of Ulp1 (Ubl-specific protease 1) expressed in E.coli. SUMO Protease cleaves in a highly specific manner, recognizing the tertiary structure of the SUMO (small ubiquitin-related modifier) rather than an amino acid sequence. The protease can be used to cleave SUMO from recombinant fusion proteins. The optimal temperature for cleavage is 30℃, however, the enzyme is active over wide ranges of temperature and pH (pH 7.0-9.0). Following digestion, SUMO Protease is easily removed from the cleavage reaction by affinity chromatography using the polyhistidine tag at the N-terminus of the protease. After cleavage, the released protein has native N-terminal amino acid sequence without additional amino acid.
Product features
1、High purity:more than 95% by SDS-PAGE assay;
2、Hige specificity: recognition the SUMO tertiary structure rather than aa sequence with native N-terminal sequence released;
3、Easily removal:the protease can be removed by one step optimized chromatography.
Recommended Conditions for Cleavage of a Fusion Protein
fusion protein(2mg/ml) 1mg
SUMO protease 10U
10 x PBS 0.1ml
Add ddH20 to 1ml
Temp(℃) 30
Time(hour) 1~2
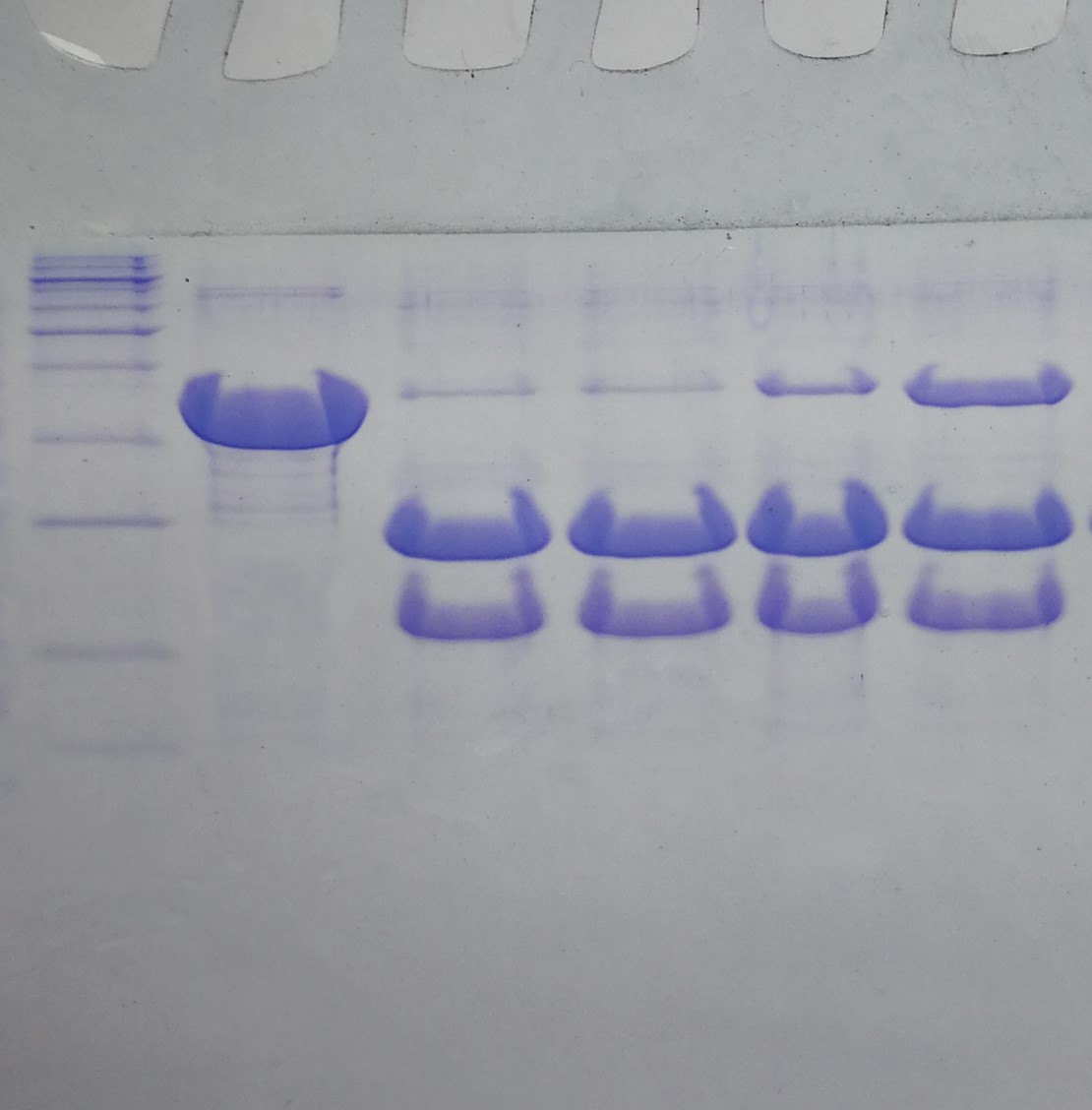
Example result
Storage
This is stable at -20℃ for more than 2 years.
Usage
This material is for laboratory purposes. NOT FOR HUMAN USE.


