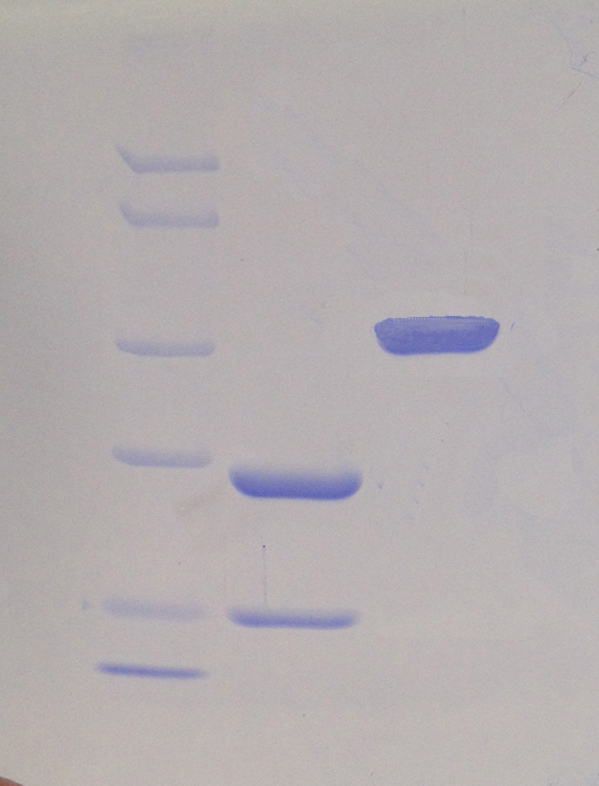
Description: Recombinant TEV Protease with GST tag, purity higher than 95%.
Product Description
Recombinant TEV Protease with GST tag (rTEV-G) is a highly purified preparation of the catalytic domain of the small nuclear inclusion a (NIa) protein encoded by the tobacco etch virus (TEV). The biological function of the protease is to proteolyse the viral polyprotein into single proteins during tobacco etch virus biogenesis. Its sequence specificity is far more stringent than that of factor Xa, thrombin. TEV protease is a very useful tool enzyme for the downstream fusion proteins cleavage which recognizes a heptapeptide consensus sequence of the general form E-Xaa-Xaa-Y -Xaa-Q-P1’(G/S), with cleavage occurring between Q and P1’. The most commonly used sequence is ENLYFQG. Many different small amino acids are tolerated in the P1’ position, allowing target genes to be released from N-terminal fusions with either a native N-terminus or with only a single amino acid substitution. It has optimally engineered and purified for enhanced stability and high specific activity over a broad temperature range. The enzyme is produced in recombinant E.coli and purified to high homogeneity with specificity of 10,000U/mg. The optimal temperature for cleavage is 30℃, however, the enzyme can be used at temperatures as low as 4℃. The rTEV-G contains GST tag. The rTEV is purified by proprietary chromatographic techniques and can be removed from protein preparation by glutathione affinity chromatorgraphy. The cleavage condition should be optimized, such as tempterature, pH and DTT.
Purity
More than 95% by SDS-PAGE analysis.
Activity
One unit is defined as the amount of enzyme that will cleave 95% of 5μg fusion protein in 12~16 hours at 4℃in 1x reaction buffer.
Recommended Enzyme Disgestion Conditions
Fusion protein 0.1mg
Recombinant TEV protease 20U
10xbuffer 10μl
Temperature(℃) 4
Total volume 100μl
Time(hour) 12~16
10x Reaction Buffer
500 mM Tris-HCl, pH 8.0
5 mM EDTA
1% Tween-20 (v/v)
10 mM DTT
Stability
Stable at -20℃ for up to two years since reported data.
Reference
Kapust RB, Tözsér J, Copeland TD, Waugh DS. The P1' specificity of tobacco etch virus protease, Biochem Biophys Res Commun. 2002 Jun 28;294(5):949-55.


